Polymers classification
The basic classification of polymers is based on their origin, and two major categories are identified: natural and synthetic ones. Natural polymers are found in nature and their structures are commonly more complicated than synthetics’ structures. Their greatest advantages include their biodegradability and their abundance. Synthetic polymers on the other hand are man made from crude oil and similar sources. Most synthetic polymers are not biodegradable although progress has been made towards manufacturing of biodegradable or partially degradable synthetic polymers in the last decade. Synthetic polymers can be tailored to reach any set of mechanical, thermal, chemical and physical properties via synthetic approaches and their raw material is quite cheap.
Natural polymers include proteins, resins, poly saccharides, poly nucleoids and other biological molecules. Synthetic polymers are categorized into thermoplastics and thermosets [or thermosetting] based on their behavior upon repeated cycles of heating and cooling. Thermoplastic polymers allow for repeated heating and cooling cycles during which they melt [heating] and they are reformed [cooling] into new shapes. Thermosets on the other hand cannot melt and be reshaped upon heating due to their 3D networking via strong covalent bonds. Both categories have unique applications in modern life. A third category, elastomers, is also met in both natural and synthetic polymers. This category exhibits significant elasticity, a property that will be discussed in forthcoming posts.
Another classification of polymers is between those that upon cooling can crystallize and those that cannot. Crystalline polymers exhibit an ordered structured of repeated micro- areas and this property affects their observed physical, chemical, mechanical and thermal properties. On the opposite side, polymers that do not crystallize from their melt but rather produce disordered structures, are called amorphous [from the Greek ‘μορφή’ that means shape]. A large number of polymers fall between these two limits, and they exhibit both crystalline and amorphous regions; these polymers are called semi crystalline and their respective properties are found mostly in the range between crystalline and amorphous polymers. Crystalline polymers demonstrate a range of melting temperature rather than a single point. This melting range [or point as it is referred in many works] is different to the crystallization range of the polymer. In monomers, these two points collide. Amorphous polymers do not exhibit a melting temperature [or range] but they do undergo a phase transition known as ‘glass transition’ at the glass transition temperature. Below glass transition temperature amorphous polymers lose their elasticity reversibly; heating above the glass transition temperature restore elasticity of the amorphous polymer. In general polymers are termed as viscoelastic materials, meaning that their observed properties can be characterized by both viscous liquids and elastic solids.
A classification of thermoplastics is based on their thermomechanical properties. It is thus commonly accepted that thermoplastics fall into the following categories:
ü Commodity polymers. This class comprises polymers of widespread commercial use such as Poly Ethylene – PE, poly Styrene, poly Vinyl Chloride – PVC, and others.
ü High performance thermoplastics. This class comprises thermoplastics with extreme mechanical and/ or thermal resistance, developed for demanding applications. Such polymers are polyimides, poly ether sulfones, and various fibers
ü Engineering thermoplastics. These thermoplastics exhibit superior mechanical properties in comparison to commodity polymers, but inferior to high performance thermoplastics. Such polymers are nylons, PET, and others. Their uses are vast, and they are used to cover every commercial application high higher requirements than commodity polymers.
Classifications can be based on any criterion of interest, and thus are not limited to the above ones. For example, based on niche applications, polymers are also classified into biomedical polymers, composite polymers, liquid crystalline, photonic polymers, molecularly imprinted polymers, and others. Each class comprises members of specific properties that make them suitable for specific applications. Biomedical polymers require high polymer purity, sterilization ability, biological compatibility, processability, specific chemical and physical properties, etc. Composites on the other hand commonly comprise additives that enhance the initial polymer’s mechanical properties. Such additives can be fibers [carbon fibers, glass fibers, nylon fibers, etc] or other compounds that create a protective ‘net’ and a ‘continuity’ of the strong material in a fashion similar of steel in reinforced cement. The ultimate goal is to substitute metallic parts due to their high cost, corrosion, erosion, undesired conductivity characteristics, and others. As one can easily guess, composites can be used in building and construction, automotive, aero motive, space and other modern applications. Imagine that a car using carbon fiber composites on the exterior, can be 50% lighter and 50% stronger that traditional metal coated cars. Liquid crystal polymers exhibit unique elasticity and impact strength properties due to their perfect orientation of chains in the melt phase. These melts sustain their organized orientation when they crystallize and thus create a highly ordered structured of long, elastic chains that result to their unique mechanical properties. Such a material is Kevlar, a material that has provided unique solutions to different segments of the industry.
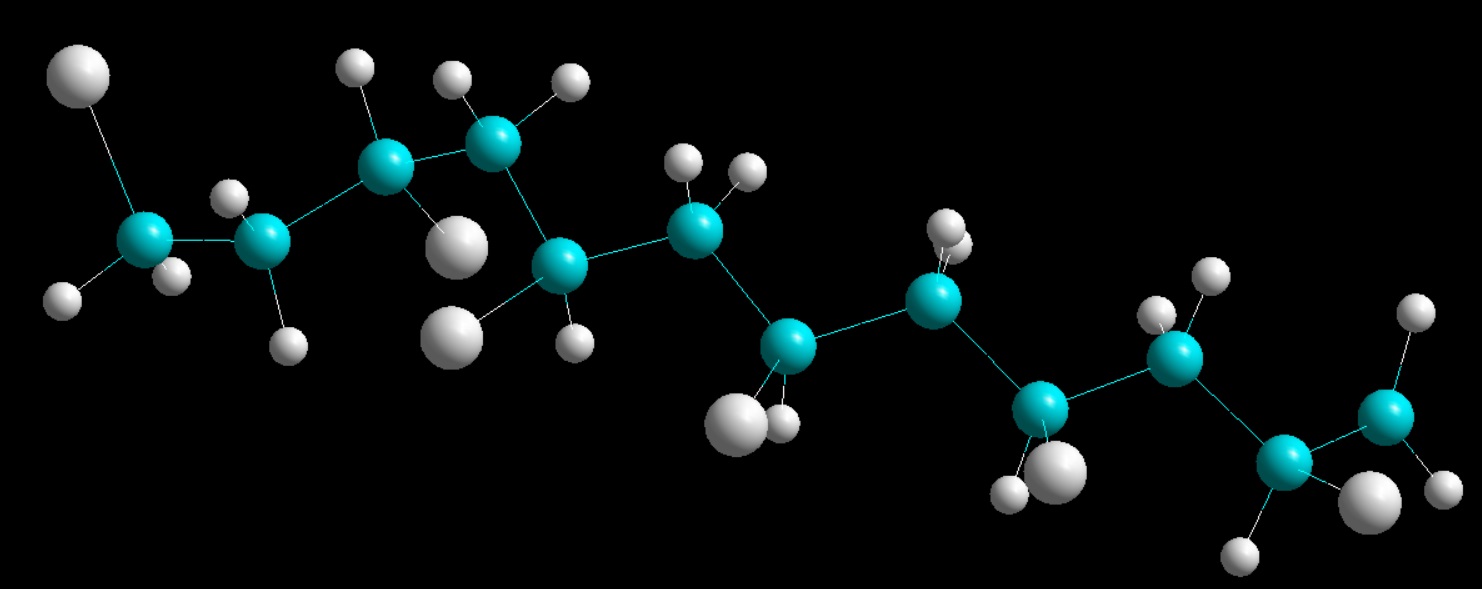
FIG: PVC structure by SinodosChemistry.com
Classification based on structure of the chains, is also used. Based on this criterion, different forms that exist are linear, branched, networked and cyclic. Each type of these classes varies greatly regarding their physical, chemical, mechanical and thermal properties. Usually linear polymers exhibit high crystallinity and all the properties that come with that. Branched polymers can be of lower quality, and networked polymers can be thermosets, non-dissolving polymers. Such polymers when added to a polar solvent can swell but it is very rare to dissolve due to the covalent 3D network that keeps the main chain system together. Copolymers are polymers that consist of more than one type of monomer. Such copolymers can be further classified into random, block, grafted, and alternating or ordered. Alternating polymers are of the structure A-B-A-B-… for their structure. Random copolymers exhibit a random, statistical distribution of the monomers in their structure of the type A-A-B-A-B-B-B-A-B- … . Block copolymers comprise blocks of pure A and B as in AAA-BBBB-AAA-BBBB-… whereas in the grafted class the chain comprises one type only and the second type of monomer is found as branches of the main chain. Copolymers will be further discussed in a forthcoming post.
