VOC removal from atmospheric environments
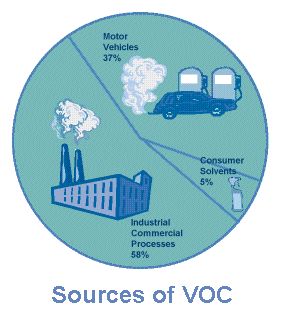
Volatile Organic Compounds [VOCs] are some of our times’ most important polluters. Many of them have been correlated to adverse health effects such as skin irritation, cyanosis, convulsions, high toxicity, carcinogenicity, kidney damages, liver damages, brain damages, asthma, and others. A group of them has been regarded as air pollutants by USEPA. They derive mainly from a range of human related activities, such as industrial processes, construction, indoor generation, and transportation. Theoretically any product or activity including polymeric compounds could be a potential VOC emitter. When referring to health adverse effects indoors, VOCs are considered as contributors to the sick building syndrome. World Health Organization [WHO] uses a classification for indoor pollutants, based solely on their respective boiling points:
- Very Volatile Organic Compounds – VVOC with boiling points in the range 50-100 °C
- Volatile Organic Compounds – VOC with boiling points from 50-100 °C to 240-260 °C and relative pressure higher than 10-2 kPa
- Semi Volatile Organic Compounds with boiling points from 240-260 °C to 380-400 °C and relative pressures in the range 10-2 – 10-8 kPa.
VOC emissions have been treated by a number of different methods, including adsorption on porous media, thermal oxidation , catalytic oxidation , biological oxidation pervaporation, photodegradation, electrocatalysis, ozonation and others. Other methodologies exist for specific cases, for example for the removal of phenol from wastewaters, such as extraction and distillation. Adsorption on porous media covers a wide range of adsorbents from Microporous carbonaceous materials [such as Activated Carbons, Carbon Nanotubes, etc] to mesoporous ordered Silicas and other ordered structures. A potential research field also exists in Metal Organic Frameworks [MOFs] and Covalent Organic Frameworks [COFs], which have been used successfully in the adsorption of other molecules such as CO2 and H2.
Adsorption is a function of porosity, pore size distribution, specific surface area, functional groups, and temperature and is governed by the enthalpy of adsorption, which is a measure of adsorbent – adsorbate interactions [ME]. Different industrial forms of porous media exist. Oxidation methods make use of different approaches for the treatment of VOCs. Thermal oxidation is the simplest case. VOCs are treated in temperatures as high as 1600 F for short times (less than a second). In the case of halogenated VOCs acid vapor is produced and subsequent treatment is required. Energy costs are high in this method and specific contaminants can reduce the overall efficiency. When using catalytic oxidation, a higher energy recovery can be achieved and a lower temperature range is utilized (less than 1000F). Commonly used catalysts for the catalytic oxidation comprise nickel, chromium, copper based solid ones and others. The issue of acid vapor production exists in this case as well. Biological reactors make use of microorganisms that use carbon as a source of energy, breaking down the organic compounds into smaller fractions that can either be used as raw materials in production or can be safely be disposed of. Biological oxidation can be combined with catalytic presence to enhance efficiency and kinetics. Review works have shed light into current advances in bio filters and bioreactors for the treatment of VOCs.
